Dehumidification of a gas by cooling
Dehumidification of a gas by cooling
In a number of processes, it is necessary to dehumidify a gas, either because they become saturated with water when they are cooled, or because we need to dry them.
For example, the Oxy-fuel processes produce fumes consisting of carbon dioxide and water, which are then separated by water condensation, which allows one to capture CO2.
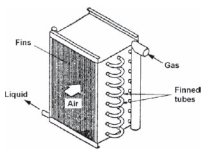
Cooling coil
To cool a moist mixture, it is passed through a special heat exchanger called a cooling coil, which can be cooled by ice water or by direct evaporation of a refrigerant (Figure above). The mixture being in contact with the cold surfaces sees its temperature decrease.
Depending on circumstances, there may be condensation or not. If there is no condensation, specific humidity remains constant and cooling can be represented by a horizontal segment oriented to the left in the Carrier chart, and vertically oriented downwards in the Mollier chart. If condensation occurs, which is very often the case, the segment is oriented to the bottom left in both charts (Figure below).
Accurate model
Theoretical perfect cooling in a cooling coil of infinite size would cool the moist mixture at the coil saturation temperature. It is customary to characterize a real process in taking this cooling as theoretical reference, introducing effectiveness e of the cooling coil and its average surface temperature ts: .e = (w2 - w1)/(wsat - w1).
A problem may arise when, in the psychrometric chart, the line from point 1 intersects the saturation curve at two points. Indeed, in this case the point calculated from effectiveness may be in the saturated zone. This is called early condensation. Two possibilities exist:
if one sets the outlet point specific humidity w2, the end point lies on the saturation curve for wsat = w2;
if we set the effectiveness, it is on the saturation curve for q'sat = q'2.
We must therefore ensure that the point is not found in the saturated zone.
External class DehumidifyingCoil.java implements this model.
Simplified model
There is an easier way, though physically approached, to resolve the problem: it consists of introducing an extraction effectiveness of the water, representing the percentage (by volume) of water condensed from that of the incoming water.
With this convention, we can recalculate the mole fraction of water in the gas exiting from that of the incoming gas.
If we give in addition the final temperature, the state of point 2 can be easily determined.
External class ColdBattery.java implements this model.
