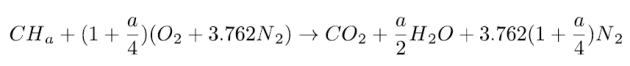Thermodynamics of combustion
Most ash-free fuels are hydrocarbons. By neglecting traces of nitrogen and sulphur, their molecule consists mainly of carbon C and hydrogen H atoms as well as some O oxygen.
By reducing the formulation to a carbon atom, the general formula of a fuel is therefore CHyOx. As the condition x <= y/2 is always checked, the CHyOx formula becomes:
CHa + x H2O
a thus represents the so-called hydrogen available for combustion related to the complete oxidation of a carbon unit.
The water in the fuel does not participate in the combustion reaction. It ends up in the fumes.
The complete combustion of a CHa formula fuel with pure oxygen would be governed by this equation: it takes 1 oxygen molecule to form CO2, and a/4 oxygen molecules to form a/2 H2O.
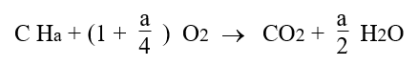
However, in energy systems, combustions are almost always carried out with air as an oxidizer.
Since dry air is approximately 79% nitrogen and 21% oxygen, the nitrogen/oxygen ratio is 79/21 = 3.762.
That's why the air can be represented by the expression O2 + 3.762 N2
A complete combustion with air of a CHa formula fuel is therefore governed by this equation:
Stoichiometric combustion is a combustion carried out with the exact amount of oxidizer to completely oxidize the fuel.
It is the one that leads to the highest end-of-combustion temperature. We will consider it as a reference combustion
This equation means that the stoichiometric combustion of a CHa fuel mole requires (1 + a/4) oxygen moles and produces 1 mole of carbon dioxide and a/2 moles of water.
If the oxidizer is air, 3.76 (1 + a/4) nitrogen moles are also involved, but because they do not react with the fuel, they end up in the burnt gases. Nitrogen is said to remain inert.
When combustion is non- stoichiometric, it can be characterized in several ways:
Either by excess air e, which as the name suggests, represents the amount of air in excess
Or by the air factor lambda, which is equal to 1 + the excess air
Or by the richness R, ratio of the number of moles (or mass) of fuel contained in a specified amount of mixture, to the number of moles (or mass) of fuel in the stoichiometric mixture.
R = 1 corresponds to the stoichiometric mixture, R < 1 to excess air, and R > 1 to excess fuel
These three factors are linked by simple relationships :
R = 1/(1 + e) and lambda = 1 + e = 1/R
We will use preferentially lambda in the following, because it is the term multiplier of air in the combustion equation.
If lambda it is greater than 1, i.e. excess air, the complete combustion with air of a CHa formula fuel is governed by this equation :
Thermoptim uses a generalization of this equation for more complex fuels than CHa.
Comparison of last two equations helps to understand what happens when air is available in excesss.
The fuel reacts with oxygen as in the stoichiometric reaction, and all excess air is found without reacting in the burnt gases.
lambda is the term that multiplies the number of air moles in the combustion equation.
For lambda = 1, the reaction is stoichiometric.
This small calculator allows you to study the influence of a and lambda on this equation. The table below shows you the number of moles of the products and their total, how the molar fractions are calculated, and their values for the chosen setting.You can test the two oxidizers used previously, oxygen or atmospheric air.
Higher and lower heating values
During combustion, the maximum amount of energy release is obtained when the water in the fumes is cooled enough to be liquefied, which requires a very low temperature. The value of the full reaction heat then takes the name of higher heating value, or HHV.
In the most general case where all the water produced remains in the vapor state, it is given the name of lower heating value or LHV
Vaporization enthalpy are far from negligible (for water, it is worth about 45 MJ/kmole at 0°C). There are therefore significant differences between the HHV and the LHV fuel values, and it is important to specify which one is being used.
The values of the LHV of fuels typically used in energy installations are quite close to each other. It can be shown that the LHV of hydrocarbons decreases from 44 MJ/kg to 40 MJ/kg as distillation products are becoming heavier.
In conclusion, therefore, to determine the energies involved in a complete combustion reaction, the heating value of the fuel is used, specifying whether it is the higher heating value (HHV) or the lower heating value (LHV).
Combustions in Thermoptim
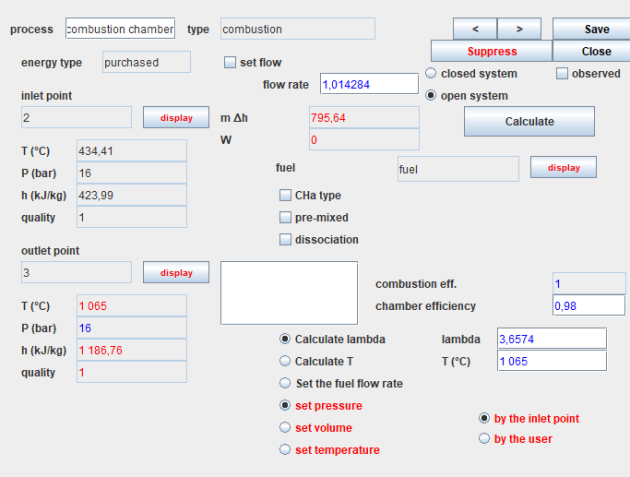
In Thermoptim, a combustion process is represented by a combustion chamber component with two inlet processes, on the one hand the oxidizer, here air at the compressor outlet, connected on the blue port, and on the other hand the fuel connected on the red port.
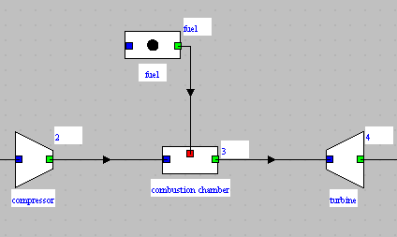
The burnt gases come out through the green port, here connected to the turbine.
The composition of the fuel is defined in the downstream point of the "fuel" process, whose subsance must be a pure or compound gas.
The natural gas composition in Montoir de Bretagne is given here :
The screen of a combustion has many parameters.
The three main methods of calculation are:
The "Calculate lambda" option determines the average air factor (lambda >= 1), based on the value of the end-of-combustion temperature set Tc.
The "Calculate T" option determines Tc from the set lambda value.
In both cases, the flow of the "fuel" process is adjusted so that the ratio of the volume flows of oxidizer and fuel is equal to the air factor.
he choice "Set fuel flow" determines lambda and Tc based on the characteristics of the fuel and the oxidizer.
The mass flow of the process being evaluated (combustion) is equal to the sum of fuel and fuel flows, which means that the combustion process behaves, hydraulically, like a flow mixer.
For other settings, when combustion takes place in an open system, the pressure set by the upstream point is generally chosen, which means that the combustion chamber is isobaric.
For simple use of Thermoptim, these settings are enough.
Incomplete combustion, dissociation, quenching temperature
We assumed in the foregoing that the combustion was complete, whereas sometimes this is not the case, especially at high temperatures, and unburned gases appear in the fumes. This is called dissociation.
If, starting from such a situation, the temperature of the reactive environment is gradually lowered, it is found that from a certain threshold its composition stabilizes and no longer varies. It is said that the reaction is quenched and the quenching temperature is called the value of this threshold.
Thermoptim can take this phenomenon into account when the dissociation mode is checked, and if it is shown on the one hand the rate of CO2 dissociation in CO, and on the other hand the value of the quenching temperature.

In this example, the CO2 dissociation rate was set at 5%, and the quenching temperature value was 900 °C.
The impact of this change in setting on the composition of the fumes is illustrated by Figures 5 and 6: Carbon monoxide CO and hydrogen H2 appear in the event of dissociation.


A combustion calculator with more setting possibilities than the small tool we presented at the beginning of this page is available here.